Helen Hunt, 60, Stuns During Her Latest Appearance, and Her Lips Become the Center of Attention

When we look at our planet from space, one color dominates. That’s why Earth is called the Blue Planet. About three-quarters of our world is covered with water. But there’s a catch.
Ninety-six point five percent of this water is trapped in the oceans. And if you remember the first time your parents took you to the seaside, drinking that water is a big no-no. So, why is ocean water salty and undrinkable?

There are two main reasons. The first is runoff water from the land. Rainwater is slightly acidic. Its pH factor is somewhere between five and five and a half. For comparison, pure water has a pH factor of seven, and the acid we find in batteries is a bit more than zero. Such rain erodes rocks when it falls on the ground. This releases ions, such as sodium and chloride. They end up in rivers in streams that eventually empty into the ocean. Living organisms remove some of the good ions, but the rest remains. Over time, this increases their concentration in the water.
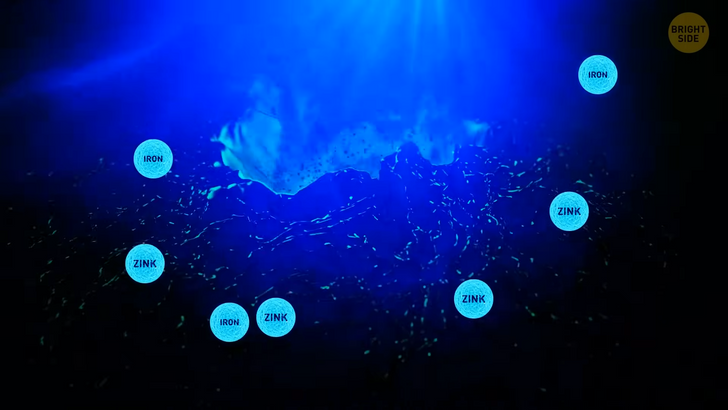
Oceans have their own salt powerhouse. Vents in the seafloor let out a hydrothermal fluid. Sounds complicated, but it’s easy to understand. Water seeps down the gaps on the ocean floor. Then the magma from the Earth’s core heats up the water. There is a chemical reaction that frees seawater from oxygen. It picks up metals, such as iron and zinc. The vents on the seafloor release this metallic water back into the ocean.

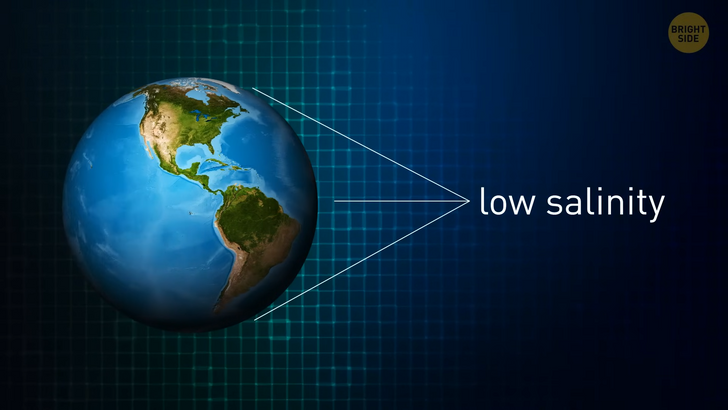
During an underwater volcanic eruption, the process speeds up. Salt and other minerals are directly released into Earth’s oceans. Over time, salt accumulates on the seafloor and forms domes. These deposits occur under dry land as well. Some places on the globe have a large number of salt domes. The Gulf of Mexico is just one example. Beneath the waves, they affect the salinity of water. Other factors that determine how salty a body of water is, include evaporation, air temperature, and precipitation [rainfall]. The general rule is that salinity is low near the equator and at the poles. All the oceans and seas in-between are likely to have high salinity. Scientists estimate that dissolved salts make up three and a half percent of the weight of the world’s seawater.
The waters that empty into the ocean, such as lakes and rivers, are fresh water. So, why is the seawater salty? To answer this question we must travel into our planet’s past. Researchers believe that primeval seas weren’t as salty as they are today. But over time, rainfall washed away the rocks on land, transporting vast amounts of salt into the oceans. The process has been going on for more than three point eight billion years. Today, some four billion tons of dissolved salts end up in Earth’s oceans every year. The input and output of salt are fairly balanced. This means that seawater’s salinity is stable.
So, why can’t we drink seawater? We already take salt into our bodies with food and drinks. It is called dietary salt [sodium chloride]. The World Health Organization recommends that humans consume no more than a teaspoon per person, per day. You shouldn’t go over that amount if you want to keep your heart healthy. Centuries ago, salted beef and pork were the standard diet of seafarers. Meat was preserved using salt. At sea, fresh fruit and vegetables would go bad after just a couple of weeks. Before refrigeration, this was the only way to keep food fresh. Pickling was another option for storing food.
The reason why we can’t drink seawater is the salt content. The percentage of this mineral in our blood is nearly four times lower than the percentage of salt in seawater. Our body simply cannot process such a high amount of the substance. When we intake salt as part of our diet, we also drink liquids. When you serve pretzels, you probably have a glass of water or juice nearby. It helps quench the thirst and keep the salt levels in check. If we drink water straight from the ocean, the exact opposite happens. We just become thirstier.
Our body absorbs both water and salt. They end up in our bloodstream. The organs responsible for getting all this salt out of our blood are the kidneys. But they need water to perform their duty. The higher the salt content, the more water they need to wash it away. When the process repeats itself several times over, you become dehydrated. This is the process of losing water from the body. And there’s another catch. You start releasing more water than you take in when you drink seawater. The difference leaves you thirstier than you were when you started drinking seawater. Not a good idea to begin with.

But some marine mammals, such as whales, seals, and even seagulls can drink water from the sea, just like we drink tap water. The kidneys of these mammals are super-efficient. Birds have special glands in their beaks that prevent salt from getting inside their bloodstream. Scientists found that the only land animal that can drink seawater is the camel. And if you ever wondered if fish drank seawater: they do. The gills and kidneys help them pump out the excess salt.
For humans to drink ocean water, it first needs to go through desalinization. This is the process of removing salt from seawater. And there’s a lot of it to take away. Estimates show that if we laid out all the sea salt across Earth’s land mass, it would be higher than the Statue of Liberty [300 feet]. That’s why desalinization on a global scale isn’t realistic. Right now, less than half a percent of the drinking water we produce [<0.5%] comes from seawater. And the demand for potable water is only going to increase. The current rate of consumption means that the demand for fresh water doubles every twenty years.
The biggest issue with desalinization is the energy cost. It takes ten times more energy than other water production methods. And the carbon footprint is huge. Large desalinization plants often need to have their own power stations. This is all because of the technology behind the process. Salt dissolves easily in water. It creates a strong chemical bond with water that is hard to break. Desalinization facilities mostly use reverse osmosis to achieve this.
Large pumps exert pressure on seawater to push it through a filter. Its membrane is so fine that each pore is a fraction of the size of a human hair. The filter allows for water molecules to pass. Larger salt molecules remain trapped in the membrane. For every quarter of a gallon of fresh water the plant generates through desalinization, there is the same amount of water that is now twice as salty. Hardly the ideal method of water purification.

The idea that humans could drink seawater isn’t new. In the mid-fourth century BCE, the famous Greek philosopher Aristotle considered using a series of filters to remove salt from water. Ships in the sixteenth century had small, portable distilleries that could boil seawater. This was merely patchwork since exposing seawater to high temperatures doesn’t make it drinkable. Such thermal processing only sterilizes the water. You would need to catch the steam that evaporates and wait for it to cool down before it’s safe to drink. This is a complex and time-consuming method that is probably not worth the effort.
Let us imagine for a second that we got rid of all the salt from the Earth’s oceans. We would get an endless supply of drinking water. But at what cost? There are millions of animal and plant species that are adapted to saltwater. These include plankton; the basis of all marine life. They wouldn’t have enough time to adapt to the new conditions. Not all fish are like the salmon which thrives both in fresh and saltwater.

The sudden switch would also have a profound effect on our planet. Since freshwater is less dense, it would immediately cause the icecap in the Arctic to sink by four inches. This would trigger the largest tidal wave the planet has ever seen. Although the idea of desalinization on a global scale sounds good on paper, we should take it with a grain of salt.