10 Thanksgiving Stories More Stuffed With Drama Than Your Holiday Turkey

Some materials on our planet have unusual and bizarre traits. Kind of like me. Prepare to be dazzled!

Number one is Aerogel. Aerogel is a solid and transparent structure. It’s like the watermelon of the science community. There’s more; aerogel is among the lightest solid materials known to human mammals. It’s really light. I mean, it’s only 3 times denser than air despite being solid. How is it made? It’s created by combining 2 materials to form a gel. Then you remove the liquid from the gel and replace it with air. This translucent material is one of the finest insulation materials. When you look at aerogel on a light background, it’s almost impossible to see. It’s like a real-world invisibility cloak. When you look at it in front of the background, it has a bluish color.
The logic is the same as why the sky is blue. Blue waves scatter the light. For the same reason, it appears transparent when you look at it under the infrared. The pores consisting of the aerogel make it an awesome option for being a thermal insulator. This is its main area of use. Since it blocks the heat very well, NASA uses it in missions. For instance, they want to use it on Mars. They don’t want the electronics to get cold on Mars at night. We cannot really see and use it in everyday life, but in the future, we can. Scientists are trying to reduce its cost and increase its durability.

Number 2 is the world’s darkest coating — Vantablack. A nanotech company has created this “black hole.” They call it that because the material is way too dark for the human eye to identify its shape and form. Imagine you walked into a room covered with Vantablack. Ugh, I would be scared. Vantablack is made out of carbon nanotubes. It absorbs 99.96% of all light that comes to it. It is grown on aluminum foil. Creases and bumps on the foil seem to disappear once it’s covered with the Vantablack. How would I look if I wore Vantablack clothes? Scientists say that I’d look like a 2-dimensional cardboard cutout. No, thanks, I like my 3-dimensional body. Anyway, this coating is intended for other stuff, like the space industry. It’s there to support air-borne cameras, telescopes, and infrared scanning systems since it’s efficient in reducing stray light.
Ultra-hydrophobic material is a cool thing that causes water to encase itself into small spheres. Here, they look like gemstones. Its superpower is being water-resistant. To make it more concrete, if you spray your windshield with it, you could drive in the rain at up to 40 miles per hour, and your windshield wouldn’t get wet. In fact, it’s not just water that turns into little balls with ultra-hydrophobic material; almost all liquids can turn into marbles with it.

Ferrofluids are similar to any kind of liquid unless they come into contact with a magnetic field. When they get magnetized, they form strange shapes. They basically do everything that usual solid magnets do, but they do it in a liquid state. They disperse randomly when the magnetic field is removed. Kind of fun to watch.
Nickel-titanium, aka Nitinol, is a metal alloy with some unusual features. What’s weird about this one is that it can always return to its original shape. It has super elasticity and can remember its original shape. Let’s assume you make a triangle out of nitinol and then bend it completely. Once you let it go, it’ll instantly start returning to its original triangle shape. Although it has other applications, Nitinol is generally used in the medical industry. A well-known example are Stents. How so? Nitinol can bend within the body when needed. It has the durability of metal, but it can always return to its original form when the pressure, which makes it warp, is discharged. The heart activates the Nitinol’s bending and shape. At some temperatures, it will bend out of its original form, and at other temperatures, it’ll turn back to its original shape.

I want to take a break from talking about all the amazing materials from the pro-science world. Let’s now pay attention to a cool substance that you can even make at home by yourself. Its fancy name is Sodium Acetate, but we can call it “hot ice” as others do. In hot ice, the process of crystallization is exo-thermic, meaning a reaction is accompanied by the release of heat. So, the result you get will be hot to the touch. It’s where the name comes from. The logic is not that complicated. Here’s the recipe for this awesome science experiment. You can create a tower of crystals or sculptures as a result of this experiment. First off, mix six tablespoons of baking soda with two liters of white vinegar. A chemical reaction will occur here. It’s going to be fizzy. Once that reaction ends, boil the mixture on medium-high heat until about 90% of the liquid is evaporated.

The sodium acetate solution contains water. You decrease the amount of water in the solution by boiling it, but of course, there is still water remaining there. When the liquid is evaporated, you should also see a crusty film formed on the top. In step 3 you should see the crystals form when the liquid evaporates, so scrape them off the sides of the pan with a spoon and add them to a small glass bowl. Set aside for the magic. Then, remove the completed solution from the heat and pour it into a small glass dish. Add a tablespoon of vinegar and stir. Fill the larger glass bowl with ice and water. Dunk a smaller glass filled with the solution into ice water until it calms down. When you cool the solution, you bring the sodium acetate to a lower temperature than the point at which it would normally become solid. Lastly, once the solution is calmed down, take a pinch of crystallized sodium acetate, and add it to the solution to create hot ice. There you go! The water molecules block the sodium acetate from forming crystals. I mean, crystals may start to form, but as a few molecules join together, the water molecules divide them again, and this is why hot ice occurs in the first place.

Hydrogels are suspended somewhere between a liquid and a solid. That’s what makes them fascinating. An everyday example would be JELL-O. A hydrogel keeps its form and doesn’t flow around objects. These are the traits of a solid object, but it also bends easily like a liquid with extremely soft pliability. I gave the example of JELL-O, but there are other types of hydrogels and other areas of use. For instance, scientists use it for biomaterial. Since it’s flexible and durable, it can be applied to the human body. Its ability to completely liquefy and fill a space catches the attention of the science community. How did it get its “wiggly” state? Hydrogels are a series of polymers. When they’re heated, the polymer proteins travel freely. When they’re cooled, those proteins harden again. Yet, they don’t go hard drastically like the time when water hardens into ice. Polymer proteins change their state seemingly smoothly.
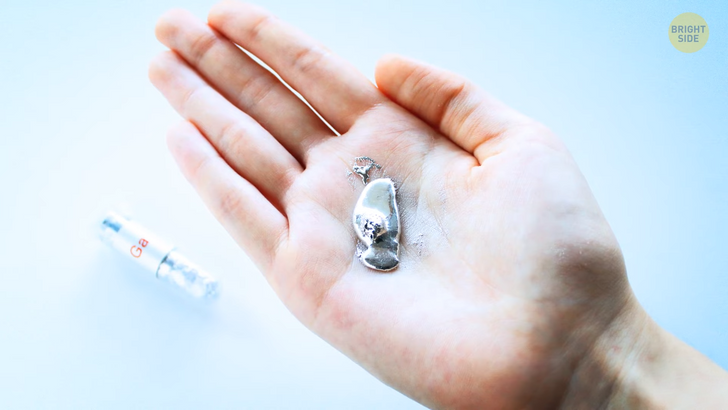
Gallium is a metallic substance resembling the liquid metal you probably saw in movies with its shiny and silvery-white color. Gallium is on this list because it has this strange characteristic. It liquefies at a fairly low temperature, around 86 degrees Fahrenheit. I mean, that’s close to room temperature in many places in summer. When you take gallium in your hand, it makes you feel like you are holding a liquid metal. There’s a bonus; you can play with metal since it’s liquid. It rolls and forms into various shapes, kind of like toys. Galium is soft even in its solid state. You can divide it into halves with a knife without needing your full arm force. This metal has many practical uses, too. For example, it’s involved in LED lights and the pharma industry. But I suspect you won’t find much of it in rocket engines.











